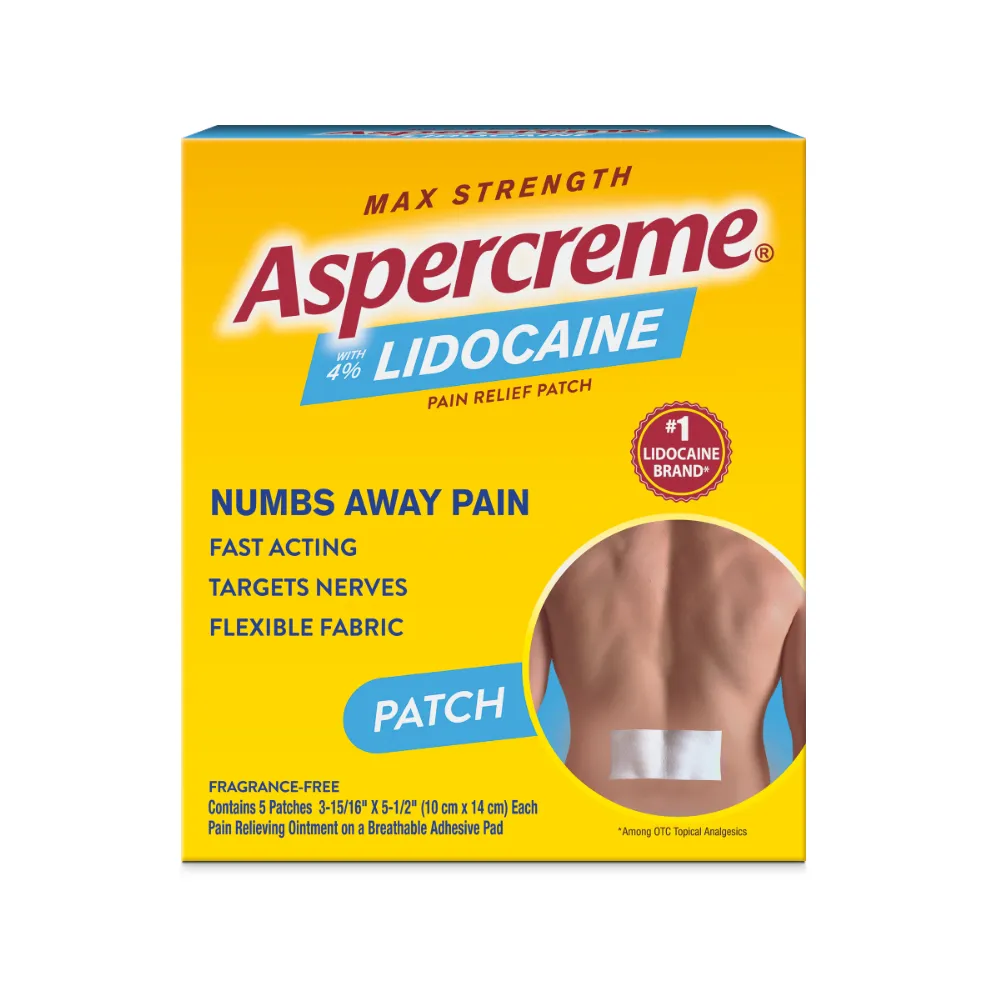
ASPERCREME® LIDOCAINE PAIN RELIEF PATCH
Quick Links: Ingredients | Directions | FAQ | Buy
The maximum-strength lidocaine available without a prescription.*
From the #1 topical lidocaine brand in the US*, Aspercreme® Lidocaine Pain Relief Patch provides fragrance-free, topical relief for minor pains.
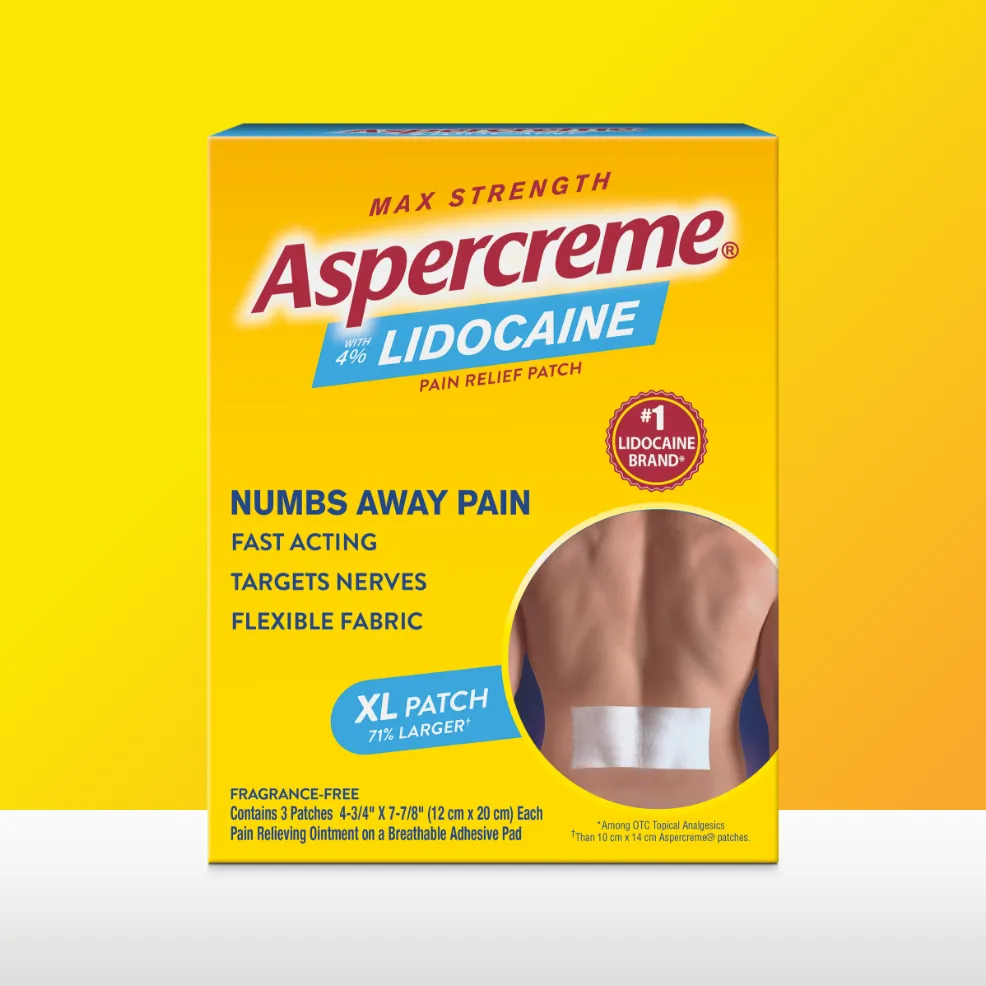
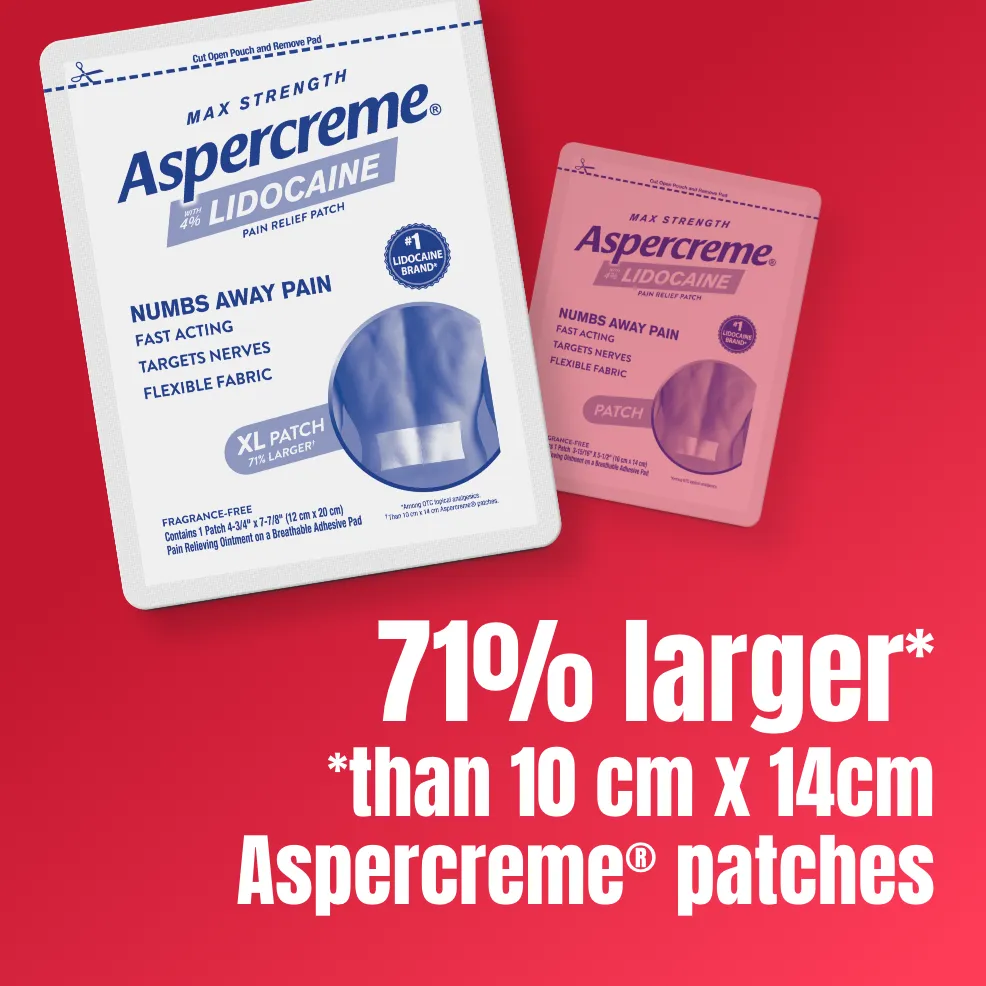
The Aspercreme® Lidocaine XL Patch is over 70% larger than our original-size patch, providing targeted pain relief to an increased surface area.
Maximum-strength lidocaine available without a prescription*
Designed to soothe aggravated nerves
Use as directed.
*Among OTC topical analgesics
Sizes:
Regular: 5-count pack | 1-count pack
XL: 3-count pack
LOCATIONS
PRODUCT INFORMATION
- clean and dry affected area
- remove backing from patch by firmly grasping both ends and gently pulling until backing separates in middle
- carefully remove smaller portion of backing from patch and apply exposed portion of patch to affected area
- once exposed portion of patch is positioned, carefully remove remaining backing to completely apply patch to affected area
- use 1 patch at a time and not more than 3 to 4 times daily
-
children under 12 years of age: consult a doctor
- on puncture wounds, cuts, irritated or swollen skin
- more than 1 patch on your body at a time or with other topical analgesics at the same time
- with a heating pad or apply local heat to the area of use
- use only as directed
- do not bandage tightly
- avoid contact with the eyes
- dispose of used patch in manner that always keeps product away from children and pets. Used patches still contain the drug product that can produce serious adverse effects if a child or pet chews or ingests this patch.
- condition worsens or symptoms persist for more than 7 days
- symptoms clear up and occur again within a few days
- redness or irritation develops
- you experience signs of skin injury, such as pain, swelling, or blistering where the product was applied
For the temporary relief of minor pain.
For external use only
Do not useIf pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children and pets. If swallowed, get medical help or contact a Poison Control Center right away.
Child-resistant packaging.
Keep carton as it contains important information.
ALL INGREDIENTS

Lidocaine HCl
Lidocaine HCl is a topical analgesic that helps ease minor pain.
aluminum glycinate, aluminum hydroxide, cellulose gum, glycerin, methyl acrylate/2-ethylhexyl acrylate copolymer, methylparaben, nonoxynol-30, polyacrylic acid, polysorbate 80, propylene glycol, silica, sodium polyacrylate, tartaric acid, titanium dioxide, urea, water
FREQUENTLY ASKED QUESTIONS
-
You may be able to use your HSA and FSA tax-preferred savings accounts to purchase certain Aspercreme® OTC products. The passage of the CARES Act by Congress includes provisions to restore OTC eligibility under tax-preferred HSA and FSA accounts. Plan details vary, so save your receipt and check with your benefits or health provider for eligibility.
-
Do not use a heating pad or ice pack while using Aspercreme® products.
-
Aspercreme® Lidocaine Pain Relief Patch is used for temporary relief of minor pain.
-
Please consult your doctor if you are pregnant or nursing before using Aspercreme® products.
-
Aspercreme® products should not be used after they expire. Check the packaging for the expiration date.
-
Do not use more than one Aspercreme® product or any other medication at the same time. Use all products only as directed. We recommend you speak to your doctor if you have additional questions.
-
We do not test Aspercreme® Lidocaine Pain Relief Patch for use on animals. Please use only as directed.
-
Aspercreme® Lidocaine Pain Relief Patch can be used for children 12 years of age and older. Please refer to the drug facts label for complete dosing instructions.
-
Aspercreme® products can be purchased without a prescription. Please visit the "Where to buy" page to find your closest retailer.
-
Aspercreme® Lidocaine Pain Relief Patches should not be reused.
-
Aspercreme® patches should not be used on an area on which another topical analgesic product has already been applied.







.png)
.png)
.png)
.jpg)
%20(1).jpg)
.jpg)

